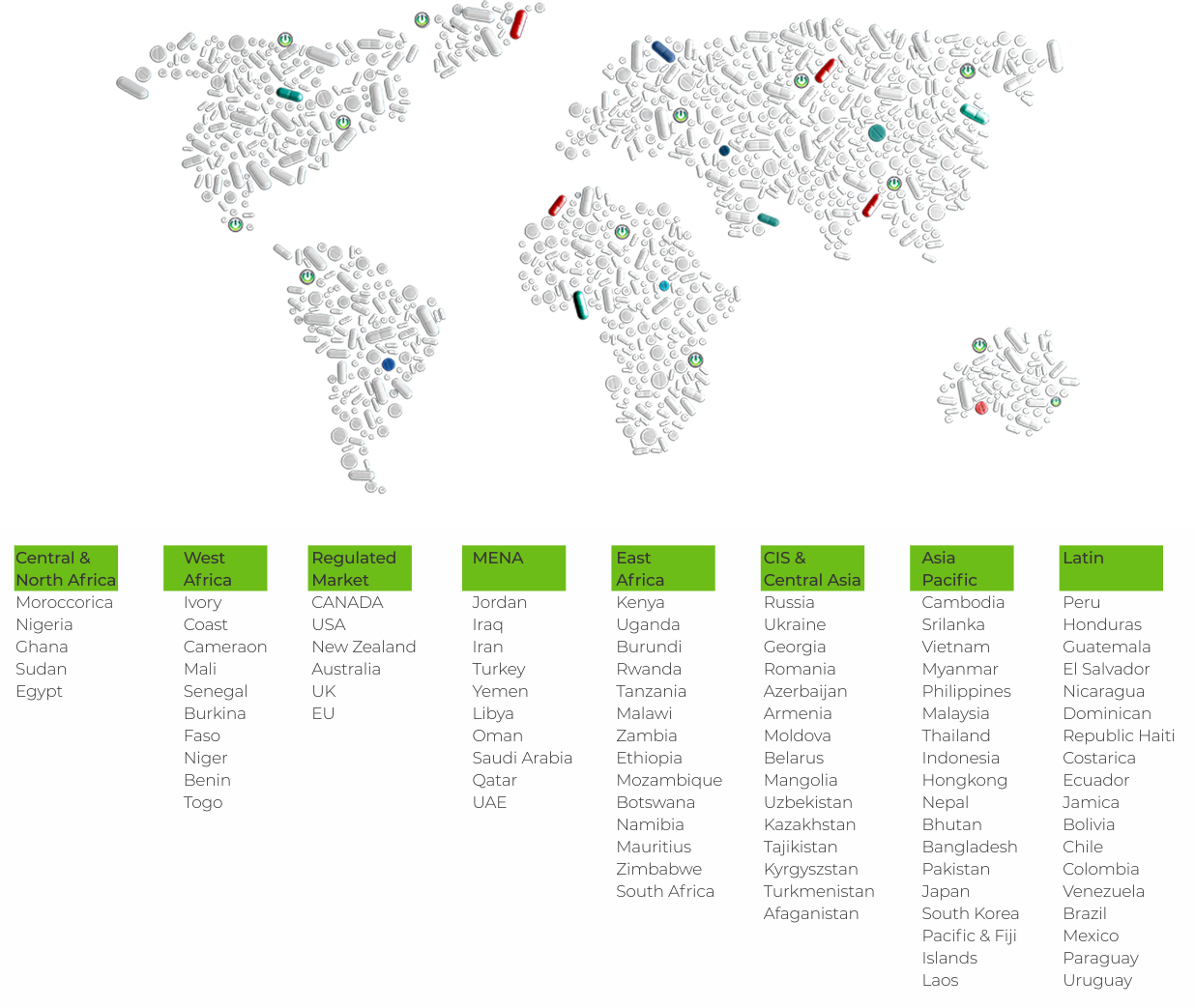
Our connect in 65+ Countries
- Selection of countries on Phased manner
- Country specific global regulatory requirements for Product dossiers & registrations.
- Global plant audits trigger and approvals.
- IP / Brand Ownership
- Identification and selection of right product mix
- Selection and appointment of right business partners / importers and distributors
- Selection and appointment of local regulatory agent
- Registration of overseas subsidiary company / representative office / branch office / local independent company
- Selection and appointment of local team
- Launch of company and products
- Promotional strategies – pre, during and post launch activities
- In licensing, Out licensing & Co development